Original paper
Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial
Abstract
A vaccine against COVID-19 is urgently needed for older adults, in whom morbidity and mortality due to the disease are increased. We aimed to assess the safety, tolerability, and immunogenicity of a candidate COVID-19 vaccine, CoronaVac, containing inactivated SARS-CoV-2, in adults aged 60 years and older.We did a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial of CoronaVac in healthy adults aged 60 years and older in...
Figures & Tables
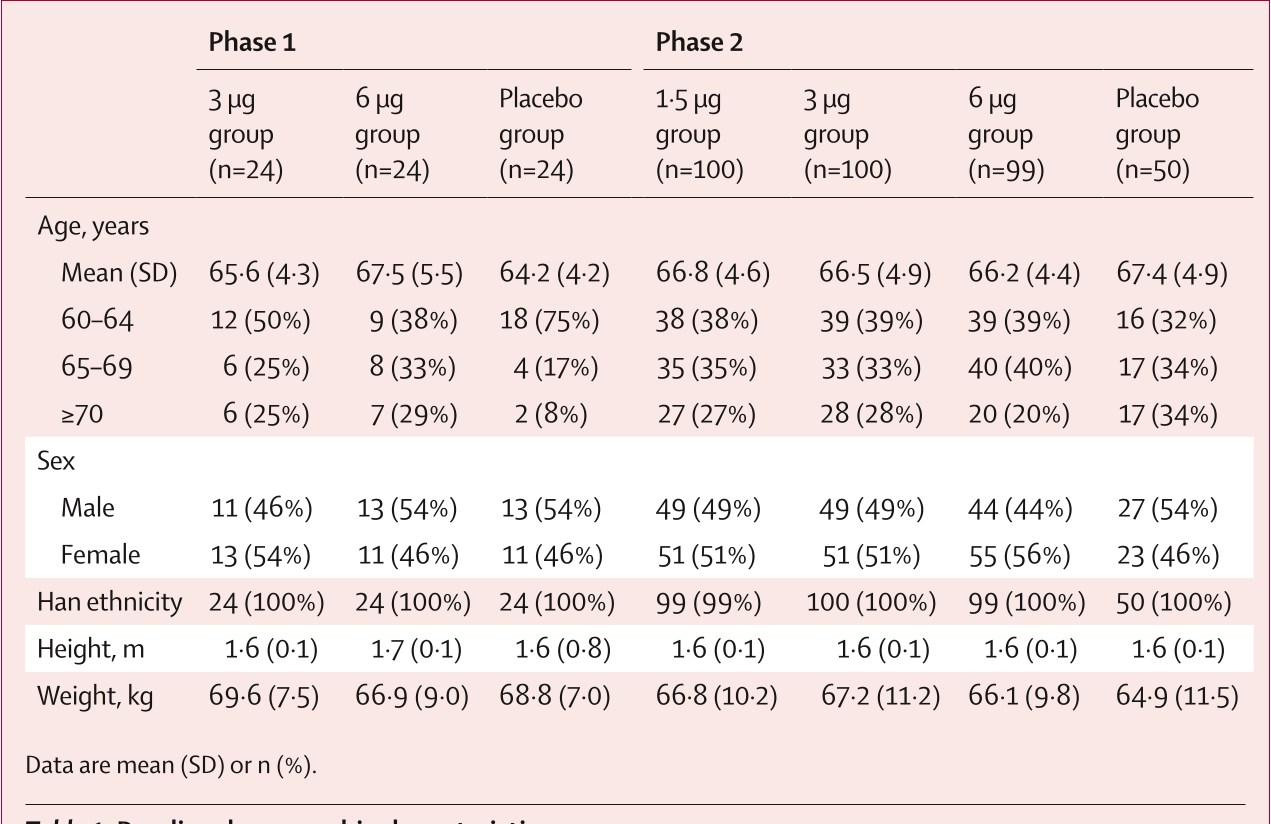
Table 1: Baseline demographic characteristics
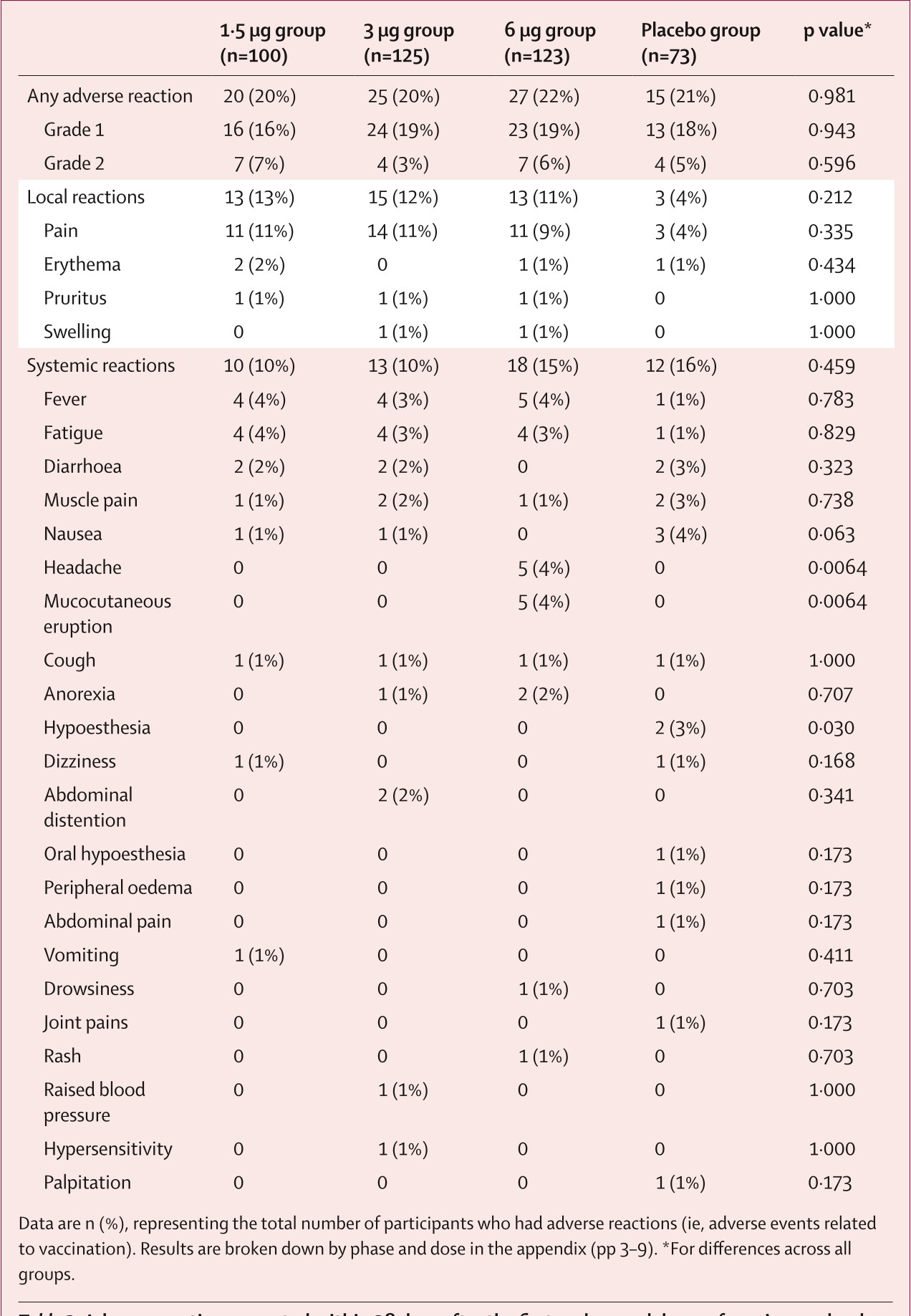
Table 2: Adverse reactions reported within 28 days after the first and second do...
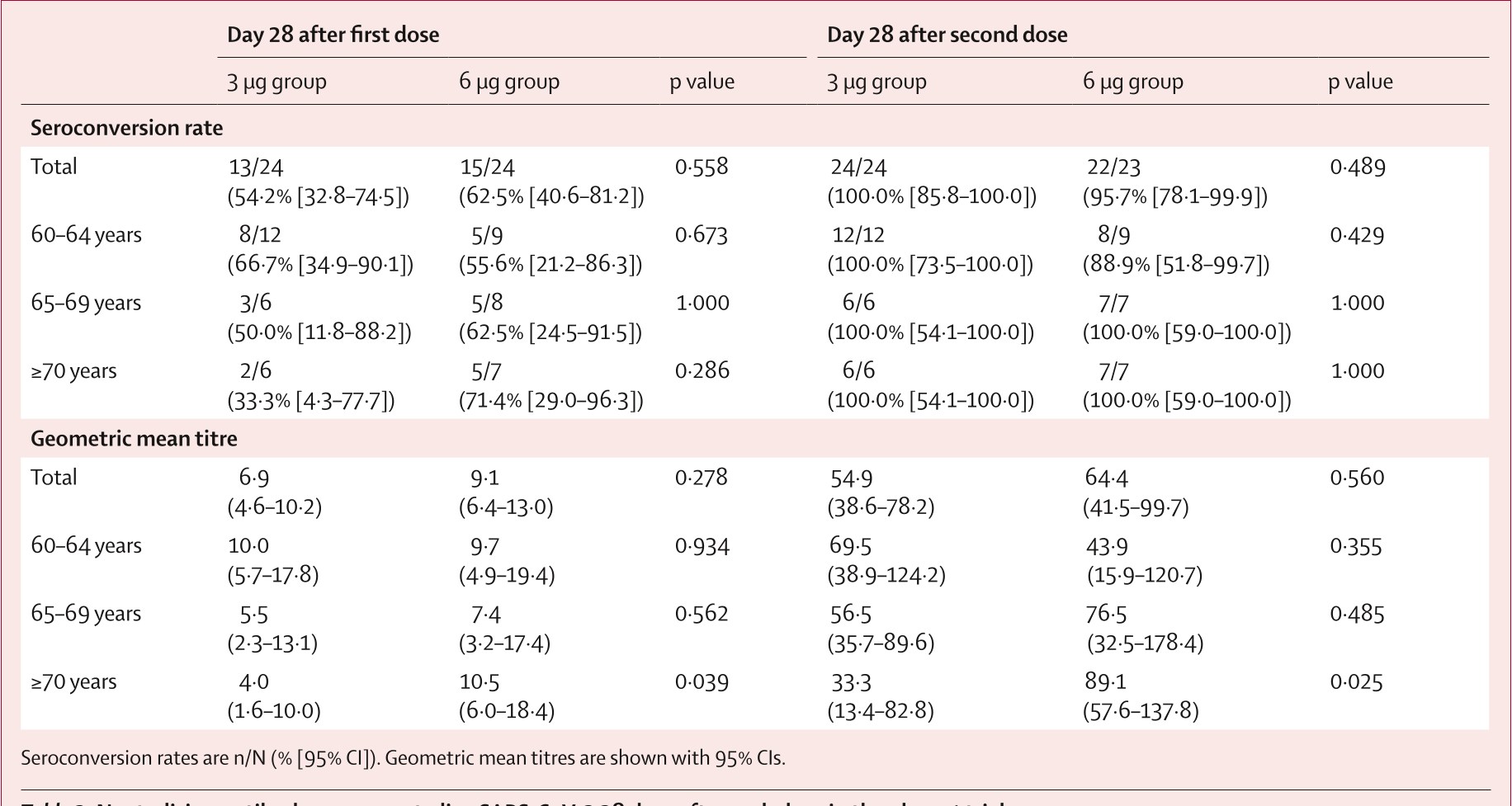
Table 3: Neutralising antibody responses to live SARS-CoV-2 28 days after each d...
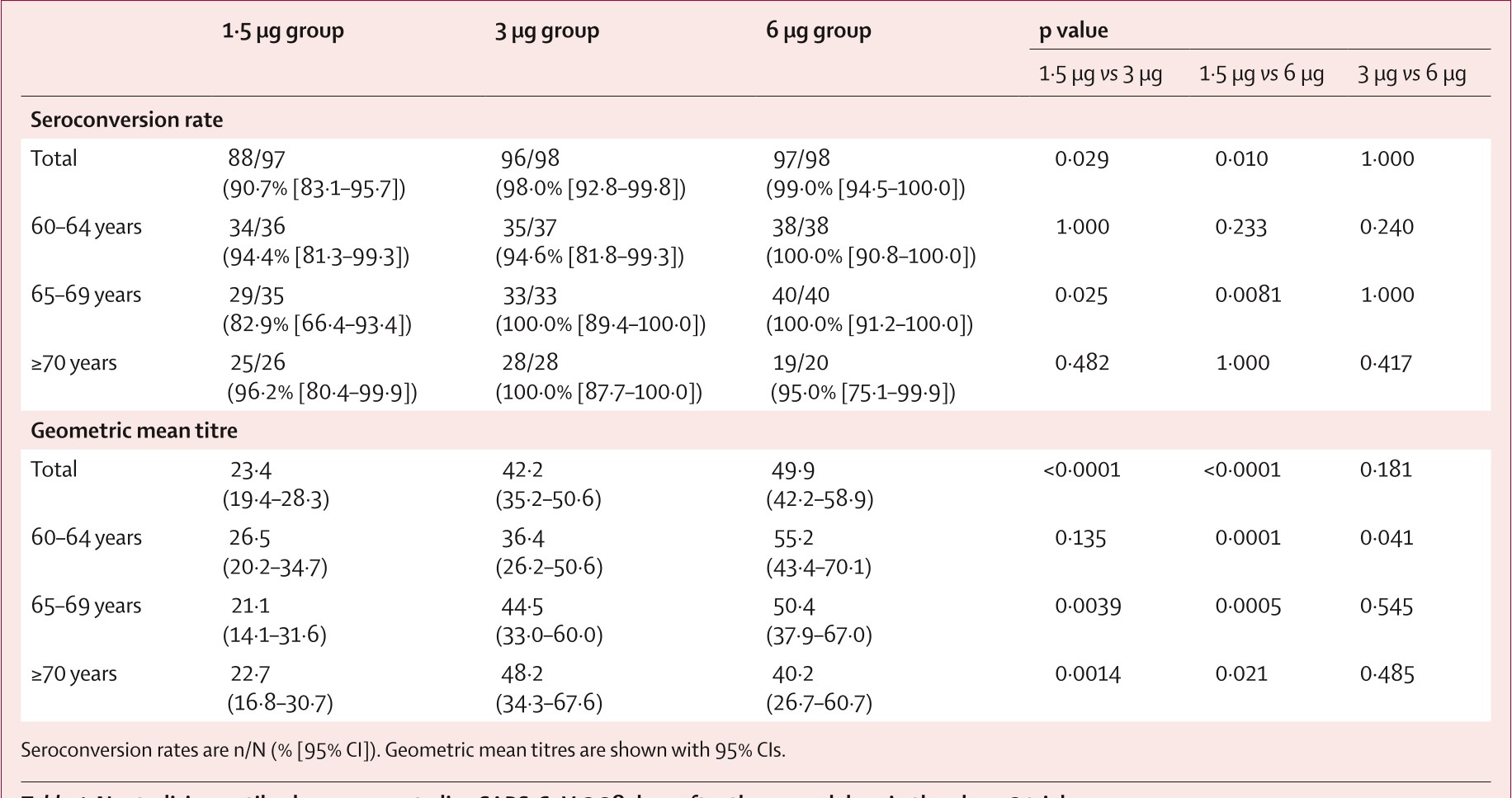
Table 4: Neutralising antibody responses to live SARS-CoV-2 28 days after the se...
Paper Details
Title
Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial
Published Date
Feb 5, 2021
Volume
21
Issue
6
Pages
803 - 812
TrendsPro
You’ll need to upgrade your plan to Pro
Looking to understand a paper’s academic impact over time?
- Scinapse’s Citation Trends graph enables the impact assessment of papers in adjacent fields.
- Assess paper quality within the same journal or volume, irrespective of the year or field, and track the changes in the attention a paper received over time.
Citation AnalysisPro
You’ll need to upgrade your plan to Pro
Looking to understand the true influence of a researcher’s work across journals & affiliations?
- Scinapse’s Top 10 Citation Journals & Affiliations graph reveals the quality and authenticity of citations received by a paper.
- Discover whether citations have been inflated due to self-citations, or if citations include institutional bias.